|
बी एड - एम एड >> बी.एड. सेमेस्टर-1 प्रश्नपत्र-IV-B - वैल्यू एण्ड पीस एजुकेशन बी.एड. सेमेस्टर-1 प्रश्नपत्र-IV-B - वैल्यू एण्ड पीस एजुकेशनसरल प्रश्नोत्तर समूह
|
5 पाठक हैं |
||||||
बी.एड. सेमेस्टर-1 प्रश्नपत्र-IV-B - वैल्यू एण्ड पीस एजुकेशन (अंग्रेजी भाषा में)
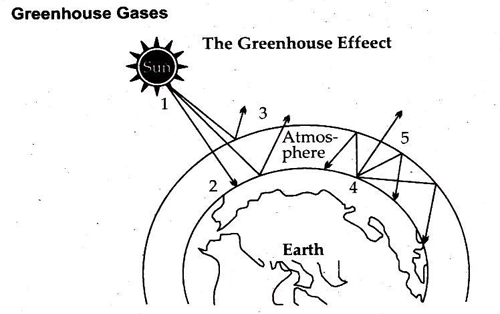
Question- Write a detailed note on Greenhouse Effect.
Answer -
Greenhouse Effect
A greenhouse is a house made of glass that can be used to grow plants. The sun’s radiations warm the plants and the air inside the greenhouse. The heat trapped inside can’t escape out and warms the greenhouse which is essential for the growth of the plants.
Greenhouse effect is the process by which radiations from the sun are absorbed by the greenhouse gases and not reflected back into space. This insulates the surface of the earth and prevents it from freezing.
Same is the case in the earth’s atmosphere. During the day the sun heats up the earth’s atmosphere. At night, when the earth cools down the heat is radiated back into the atmosphere. During this process, the heat is absorbed by the greenhouse gases in the earth’s atmosphere. This is what makes the surface of the earth warmer, that makes the survival of living beings on earth possible.
(1) Carbon dioxide (CO₂) :
Carbon dioxide enters the atmosphere through burning fossil fuels (coal, natural gas, and oil), solid waste, trees and other biological materials, and also as a result of certain chemical reactions (e.g., manufacture of cement). Carbon dioxide is removed from the atmosphere (or “sequestered”) when it is absorbed by plants as part of the biological carbon cycle.
(2) Methane (CH₄) :
Methane is emitted during the production and transport of coal, natural gas, and oil. Methane emissions also result from livestock and other agricultural practices, land use and by the decay of organic waste in municipal solid waste landfills.
(3) Nitrous oxide (N₂O) :
Nitrous oxide is emitted during agricultural, land use, industrial activities, combustion of fossil fuels and solid waste, as well as during treatment of wastewater.
(4) Fluorinated gases :
Hydro fluorocarbons, perfluoro carbons, sulfur hexa fluoride, and nitrogen trifluoride are synthetic, powerful greenhouse gases that are emitted from a variety of industrial processes. Fluorinated gases are sometimes used as substitutes for stratospheric ozone-depleting substances (e.g., chlorofluorocarbons, hydro chlorofluorocarbons, and halons). These gases are typically emitted in smaller quantities, but because they are potent greenhouse gases, they are sometimes referred to as High Global Warming Potential gases (“High GWP gases”).
(5) Water vapour :
Water vapour is the most potent greenhouse gas in Earth’s atmosphere, but its behaviour is fundamentally different from that of the other greenhouse gases. The primary role of water vapour is not as a direct agent of radiative forcing but rather as climate feedback. That is, as a response within the climate system that influences the system’s continued activity. This distinction arises because the amount of water vapour in the atmosphere cannot, in general, be directly modified by human behaviour but is instead set by air temperatures. The warmer the surface, the greater the evaporation rate of water from the surface. As a result, increased evaporation leads to a greater concentration of water vapour in the lower atmosphere capable of absorbing infrared radiation and emitting it back to the surface.
(6) Surface-level ozone :
The next most significant greenhouse gas is surface, or low-level, ozone (O₃). Surface O₃ is a result of air pollution; it must be distinguished from naturally occurring stratospheric O₃, which has a very different role in the planetary radiation balance. The primary natural source of surface O₃ is the subsidence of stratospheric O₃ from the upper atmosphere. In contrast, the primary anthropogenic source of surface O₃ is photochemical reactions involving the atmospheric pollutant carbon monoxide (CO). The best estimates of the natural concentration of surface O₃ are 10 ppb, and the net radiative forcing due to anthropogenic emissions of surface O₃ is approximately 0.35 watt per square metre. Ozone concentrations can rise to unhealthy levels (that is, conditions where concentrations meet or exceed 70 ppb for eight hours or longer) in cities prone to photochemical smog.
Causes of Greenhouse Effect
The major causes of the greenhouse effect are:
(1) Burning of Fossil Fuels :
Fossil fuels are an important part of our lives. They are widely used in transportation and to produce electricity. Burning of fossil fuels releases carbon dioxide. With the increase in population, the utilization of fossil fuels has increased. This has led to an increase in the release of greenhouse gases in the atmosphere.
(2) Deforestation :
Plants and trees take in carbon dioxide and release oxygen. Due to the cutting of trees, there is a considerable increase in the greenhouse gases which increases the earth’s temperature.
(3) Farming :
Nitrous oxide used in fertilizers is one of the contributors to the greenhouse effect in the atmosphere.
(4) Industrial Waste and Landfills :
The industries and factories produce harmful gases which are released in the atmosphere.
Landfills also release carbon dioxide and methane that adds to the greenhouse gases.
Effects of Greenhouse Effect
The main effects of increased greenhouse gases are:
(1) Global Warming :
It is the phenomenon of a gradual increase in the average temperature of the Earth’s atmosphere. The main cause for this environmental issue is the increased volumes of greenhouse gases such as carbon dioxide and methane released by the burning of fossil fuels, emissions from the vehicles, industries and other human activities.
(2) Depletion of Ozone Layer :
Ozone Layer protects the earth from harmful ultraviolet rays from the sun. It is found in the upper regions of the stratosphere. The depletion of the ozone layer results in the entry of the harmful UV rays to the earth’s surface that might lead to skin cancer and can also change the climate drastically.
The major cause of this phenomenon is the accumulation of natural greenhouse gases including chlorofluorocarbons, carbon dioxide, methane, etc.
(3) Smog and Air Pollution :
Smog is formed by the combination of smoke and fog. It can be caused both by natural means and man-made activities.
In general, smog is generally formed by the accumulation of more greenhouse gases including nitrogen and sulfur oxides. The major contributors to the formation of smog are the automobile and industrial emissions, agricultural fires, natural forest fires and the reaction of these chemicals among themselves.
(4) Acidification of Water Bodies :
Increase in the total amount of greenhouse gases in the air has turned most of the world’s water bodies acidic. The greenhouse gases mix with the rainwater and fall as acid rain. This leads to the acidification of water bodies.
Also, the rainwater carries the contaminants along with it and falls into the river, streams and lakes thereby causing their acidification.
Runaway Greenhouse Effect
This phenomenon occurs when the planet absorbs more radiations than it can radiate back. Thus, the heat lost from the earth’s surface is less and the temperature of the planet keeps rising. Scientists believe that this phenomenon took place on the surface of Venus billions of years ago.
This phenomenon is believed to have occurred in the following manner:
-
A runaway greenhouse effect arises when the temperature of a planet rises to a level of the boiling point of water. As a result, all the water from the oceans converts into water vapour, which traps more heat coming from the sun and further increases the planet’s temperature. This eventually accelerates the greenhouse effect. This is also called the “positive feedback loop”.
-
There is another scenario giving way to the runaway greenhouse effect. Suppose the temperature rise due to the above causes reaches such a high level that the chemical reactions begin to occur. These chemical reactions drive carbon dioxide from the rocks into the atmosphere. This would heat the surface of the planet which would further accelerate the transfer of carbon dioxide from the rocks to the atmosphere, giving rise to the runaway greenhouse effect.
In simple words, increasing the greenhouse effect gives rise to a runaway greenhouse effect which would increase the temperature of the earth to such an extent that no life will exist in the near future.
|
|||||













